
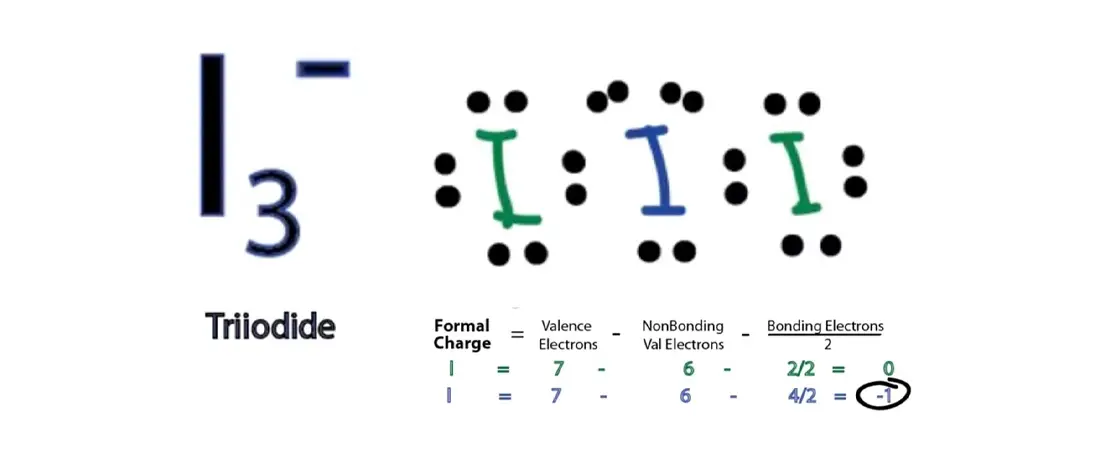
Let's say you had the formula of water, `H_2O`, and wanted to know its molecular geometry. In other words, by drawing out the Lewis structure of a molecule, one can determine the molecule's 3d orientation.

We're going to discuss each one individually, but note that you can determine the molecular geometry of a molecule solely by the number of bonds and lone pairs around the central atom. The following chart provides the different molecular geometries and the conditions in which they arise. This will become important when discussing bond angles.
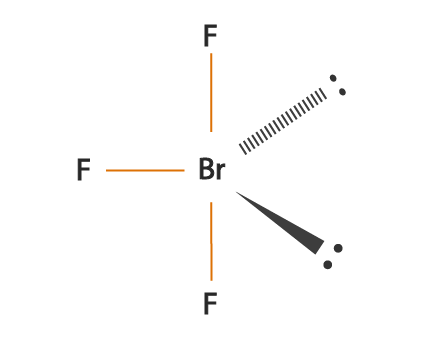
If you substitute a single bond with a lone pair, the lone pair will repel the other electron groups away from it more than the single bond would.
One key point is that lone pairs take up more room than single bonds do. The lone pair is usually not shown in 3d models and thus you have to visualize it yourself. This is why `NH_3` has a tri-pod shape: the lone pair on top of `N` pushes the other electrons (in the bond) away from it. The basic idea behind VSEPR is that electron pairs will repel each other. The only difference is the number of electrons on the central atom: `N` has a lone pair whereas `B` does not. Take a look at the Lewis structures of `NH_3` and `BF_3`. Molecular geometry is determined by the arrangement of valence electrons. The reason behind this is that the two structures have a different number and arrangement of valence electrons. This is the case of `BF_3` and `NH_3`: both have the general formula `AB_3`, but `NH_3` on the left is a tri-pod shape whereas `BF_3` on the right is a 3-legged starfish shape. In other words, two molecules with the general formulas `AB_3` may look completely different in real life: one may be a pyramid whereas the other may be completely flat. Valence Shell Electron Pair Repulsion (VSEPR) is a theory that states that the 3d orientation, also known as the molecular geometry, of a molecule is not dependent on its chemical formula but on the repulsion of valence electrons.


 0 kommentar(er)
0 kommentar(er)
